Gather a Strong Joint Force for High-quality Development | the First Meeting of the Academic Committee of Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center Was Grandly Hel
On December 31, 2022, the First Meeting of the Academic Committee of Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center was grandly held in Beijing. This meeting brought authoritative experts from various disciplines at home and abroad together, focusing on the "new model of intelligent development of improved new drugs" for in-depth discussion and exchange. Participants fully discussed and determined the strategies for the problems, risks and challenges in the establishment, pharmacology and clinical research of new modified drugs in many fields such as children and cancer in China. Besides, They also discussed the application of Cutting-edge Drug Delivery Technology, AI Simulation Data Modeling, IVIVR PK/PD Bridging and other technologies in new drug research and development,aiming to propose efficient and effective comprehensive solutions for the mission of "people-oriented, unmet clinical needs of precision medicine" for patients worldwide.

More than 40 experts attended the meeting online and offline, including Erdan Dong, Academician of CAE, Director of the Institute of Cardiology of Peking University, Director of the Key Laboratory of Molecular Cardiology of the Ministry of Education; Guanghui Ma, Academician of CAS, Researcher of the Institute of process engineering of CAS, Director of the National Key Laboratory of Biochemical Engineering, Director of the National Research Center for Biochemical Engineering Technology (Beijing), and Chairman of the Academic Committee of the Joint Research and Development Center; Professor Jiancheng Wang, Director of Peking University Health Science Center; Professor Chunli Song, Vice President of Peking University Third Hospital; Director of Scientific Research Division, Researcher Liying Yan; Deputy Director of Scientific Research Division, Haiyan Li;Mr. Wallace Tao, Chairman of Leadingpharm; Ms. Julie Gao, President of Leadingpharm;Mr. Jianlei Kang, Vice President of Leadingpharm; Professor Tongyan Han, Member of the Academic Committee of the Joint Research and Development Center and Director of Pediatrics Department of the Third Hospital of Peking University;Baoshan Cao, Director of the Department of Cancer Chemotherapy and Radiology.The meeting was co-chaired by Haiyan Li, Director of the Drug Clinical Trial Institution of Peking University Third Hospital, and Dongyang Liu, Deputy Director of the Drug Clinical Trial Institution.

Academician Erdan Dong of CAE delivered a speech at the meeting. He said that the Ministry of Science and Technology clearly pointed out in the 2022-2023 Action Plan for Improving the Technological Innovation Ability of Enterprises that the country will further strengthen the precise implementation of policies, guide enterprises to strengthen the key core technology research, and support enterprises to carry out basic and cutting-edge research on forward-looking layout. In this special period, all clinical experts, pharmaceutical research and development teams, enterprise representatives and so on, have overcome various difficulties to get together for the same goal and vision, which is the embodiment of mission and sentiment. This meeting is really a good example of the combination of pharmacy and clinical medicine, as well as the combination of medical treatment and enterprise. On the road of innovative drug research and development, we believe that with this multi-level cooperation between government, industry, university, research and application, we can accelerate the transformation process of innovative results and benefit the majority of patients.
Jiancheng Wang, Director of the Scientific Research Division of Peking University Medical Department, pointed out that technology, talent and inclusion are the three main elements of innovation. Over the years, under the leadership of Academician Jie Qiao, Peking University Third Hospital has actively integrated into the national scientific and technological innovation development strategy, actively explored measures of transformation of medical scientific and technological achievements, including system construction, institution construction and team construction. The hospital established Innovation Research Institute, Clinical Medical Center, Innovation and Transformation Center, Joint Research and Development Center of Colleges and Enterprises and so on. All these have effectively promoted the innovation and transformation of scientific and technological achievements of the hospital, and provided a good reference for the construction of the common technology supply system and the transformation system of scientific and technological achievements in the process of scientific and technological achievements transformation. The platform of Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center will focus on specific groups such as children and tumors, go deep into the clinical frontline, effectively tap the unmet clinical needs, give full play to the important role of drug delivery technology in the research and development of improved new drugs, and make concerted efforts to break through key technical bottlenecks, and provide new ideas and new measures for the intelligent development of improved new drugs.

Chunli Song, Vice President of Peking University Third Hospital, has high hopes for the joint research and development center. He said that the establishment of the joint research and development center is a major change from an industrial mass production to a people-oriented precision medical treatment, and a major change from "the amount of drugs is basically guess, the use of drugs is basically break" to the precise and personalized use of drugs for the elderly, children and other special groups. He hope that all academic members will actively contribute their opinions and suggestions to truly build the improved new drug intelligence platform into an international first-class brand project.

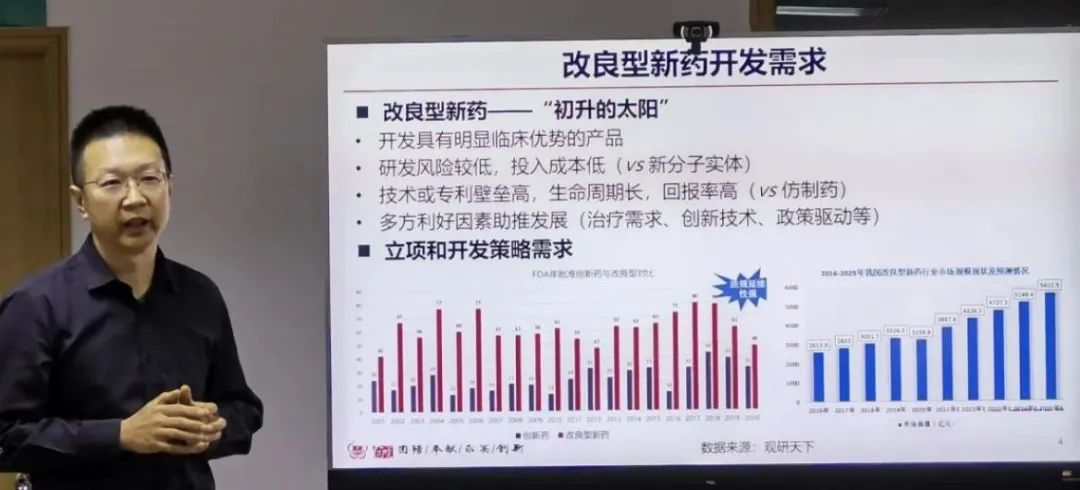
"The research and development of both innovative drugs and generic drugs should be guided by the clinical needs of patients, while taking into account the commercial transformation ability and commercial value. The regulatory strategy, research and development strategy, market prediction and legal feasibility are indispensable as the four supporting points. By analyzing the number and sales data of innovative drugs and improved new drugs in the past five years, we can see that compared with the development of original new drugs, on the basis of known active ingredients,the structure, dosage form, prescription process, route of administration, indications and so on have been optimized, and have become the main force of new drug research and development with its clear mechanism of action, clinical efficacy, short development cycle, high rate of return, and huge market space. By analyzing the listed products, we can find that the market of the products is not in direct proportion to their technical difficulties, and drug delivery is the key to the proprietary drugs. " Liang Zhao, director of FDA/CDER/Department of Modeling and Simulation of Generic Drug Evaluation, made the theme report of Modeling Advance for Drug Delivery, which provided us with new thinking on the development of improved new drugs.
Deputy Director of the Drug Clinical Trial Institution of Peking University Third Hospital, Director of the Joint Research and Development Center, Dongyang Liu, made the theme report of Opportunities and Challenges of the College's New Drug Development: Our Task Discussion. He introduced the research and development of the joint research and development platform and the expert team, the platform operation mode, project initiation and development strategy, as well as the expected scientific research achievements of the platform in the short, medium and long term in the future. The joint research and development center will gather clinicians, academic committee experts, Leadingpharm and drug clinical trial institutions to serve as an efficient transformant and research model innovation team, which will follow the guidance of the unmet clinical needs, form a new model of intelligent development of improved new drugs, explore and incubate new products, new technologies and new strategies, and improve the hospital's development and transformation ability and clinical discipline innovation ability, jointly enable and accelerate the innovation and development of improved new drugs in China.
Subsequently, the project leaders of the Joint Research and Development Center reported on the proposed projects of the platform respectively. project leaders including Member of the Academic Committee, Academician Guanghui Ma of the Chinese Academy of Sciences; Professor Bei Hu of the former Clinical Pharmacology Research Center of Peking Union Medical College Hospital; President Qian Feng of the School of Pharmacy of Tsinghua University, Professor Xianrong Qi of the School of Pharmacy of Peking University; Researcher Aiping Zheng of the Institute of Toxicology and Drugs of the Academy of Military Medical Sciences; Professor Lin Shen of Beijing Cancer Hospital; Chief Physician Kunling Shen of Beijing Children's Hospital of Capital Medical University, and Professor Defang Ouyang of the Institute of Chinese Medical Sciences, University of Macau respectively put forward constructive opinions and suggestions on the technical methods and strategies of the proposed project, pointing out the direction for the development and construction of the follow-up platform.

Ms. Gao Shijing, President of Leadingpharm, put forward suggestions and ideas on the technical methods and strategies of the proposed project, and expressed her original intention of establishing a joint research and development center, as well as her expectations for the future.
Universities and scientific research institutes are like the fertile soil of innovation, scientific research teams and project teams led by academicians and academic leaders are like the seeds of innovation, enterprises(including state-owned enterprises, central enterprises and large private groups) the carrier of innovation, the efficiency of innovation are CXO enterprises that participate in the whole process, the beneficiaries of innovation results are hospitals, and the beneficiaries of innovation are vast numbers of patients. Leadingpharm is willing to try to truly implement the innovation research center integrating government, industry, education, research and application.
Innovative medicine is a systematic project that requires technology, capital, policy, time and feelings. CXO enterprises are an important force in the process of new drug development and the guarantee of efficiency and professionalism. Since its establishment for 18 years, Leadingpharm has been innovating, developing generic drugs, improved new drugs and innovative drugs in innovative ways, and has accumulated a lot of experience in pharmaceutical and clinical research in relevant fields. Leadingpharm hopes to actively integrate innovative research and development, starting from the top-level design of topic selection and project approval, risk assessment, and construction from project to platform. On one hand, we hope to turn the project experience accumulated by enterprises for many years into data assets with the in-depth cooperation model of Government-Industry-University-Research-Users Cooperation, and realize the maximization of value in the application stage. On the other hand, we hope to achieve breakthroughs in technology and innovation under the joint guidance and participation of various industry experts, and help the whole industry develop to a higher level. As a research and development company covered by CXO Entire Industrial Chain, we hope to be a reliable partner of innovation researchers, truly give full play to the advantages of enterprises in the transformation application and industrial chain closed-loop, improve the efficiency of innovation research and transformation, accompany the growth of the project throughout the whole process, help the innovation results to be listed early and benefit the majority of patients. It is believed that with the joint efforts of various parties, the system engineering of intelligent development of improved new drugs will be completed with high quality and efficiency, and the mission of "people-oriented, precision medical care to meet the unmet clinical needs" of the pharmaceutical industry will be shouldered, and a new benchmark for the development and cooperation of innovation-driven integration of Government-Industry-University-Research-Users Cooperation will be set. Finally, she expressed her gratitude to all the experts attending the meeting, especially to Director Haiyan Li and Director Dongyang Liu, who organized and presided over the first meeting of the Academic Committee.
Subsequently, the representative of the enterprise, Vice President of CSPC Group, Jinheng Hao; Director of Huluwa, Qibo Tang and Director of COSCI, Jiang Xin, said that they would communicate and cooperate more. Tongyan Han, Director of Pediatrics Department, said that she hoped to better solve the medication dilemma of children; Director of the Scientific Research Division of Peking University Third Hospital Liying Yan and Deputy Director of the Scientific Research Division of Peking University Third Hospital Haiyan Li, congratulated the joint research and development center and said that they would fully support the development of the joint research and development center, and encourage multiple joint clinical department experts. Finally, Director Haiyan Li, on behalf of the hospital, expressed sincere gratitude to the experts of the academic committee, partners of Leadingpharm and corporate guests, and hoped that the Research and Development Center could become the national team of intelligent development of improved new drugs in the future and develop good drugs that really solve the problems of patients!
The establishment of Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center is to respond to the guidance and call of Academician Jie Qiao of the Medical Department of Peking University, integrate multiple resources, establish an advanced drug development platform with joint efforts of hospitals and enterprises, integrate basic research, clinical research and clinical innovation and transformation, and jointly build a world-class research hospital.
Leadingpharm has been laid out for three years in the strategic plan for the development and construction of the improved new drug R&D sector, and has achieved phased results. The establishment of the Improved New Medicine and Intelligent Development Joint Research and Development Center with Peking University Third Hospital is an important step of Leadingpharm in the strategic deployment of high-end pharmaceutical innovation and transformation, which will further consolidate the innovative development and promote the progress of the industry.
Introduction of Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center
Peking University Third Hospital·Leadingpharm Improved New Medicine and Intelligent Development Joint Research and Development Center was funded by Leadingpharm and jointly established with the Innovation Research Center of Peking University Third Hospital.
The center will serve as an efficient transformant and research model innovation team of improved new drugs. It will take improved new drugs such as children and cancer as the experimental field, take unmet clinical needs as the guidance, integrate new technologies such as modeling and simulation, domestic and foreign frontier preparation development, and gather the forces of clinical doctors, academic committees, Leadingpharm and drug clinical trial institutions to explore and incubate new products, new technologies and new strategies, Improve the development and transformation ability of hospitals and the innovation ability of clinical disciplines, and jointly empower and accelerate the innovative development of improved new drugs in China. The center implements the director responsibility system of the joint research and development center under the leadership of the academic committee. Academician Guanghui Ma of the Chinese Academy of Sciences served as the chairman of the academic committee, Professor Bei Hu of the former Clinical Pharmacology Research Center of Peking Union Medical College Hospital served as the deputy chairman, and invited Professor Lin Shen of the Beijing Cancer Hospital, Chief Physician Kunling Shen of the Beijing Children's Hospital of the Capital Medical University, Feng Qian, Dean of the School of Pharmacy of Qinghua University, Professor Xianrong Qi of the School of Pharmacy of Peking University, and Researcher Aiping Zheng of the Institute of Toxicology and Drugs of the Academy of Military Medical Sciences Professor Ouyang Defang of the Chinese Academy of Medicine of the University of Macau served as a member of the committee, and was responsible for recommending the main research direction of the Joint Center, reviewing the research plans of the Joint Center, regularly assessing the research progress, reviewing the research results, and guiding and supporting the development of improved new drugs. The management committee of the Joint Research and Development Center is responsible for its daily management, with Haiyan Li, the director of the clinical drug trial institution of Peking University Third Hospital, as the consultant, Dongyang Liu, the director, Tongyan Han, the chief physician of pediatrics, and Baoshan Cao, the chief physician of the Department of Cancer Chemotherapy and Radiology, as the deputy director, Zhenzhen Yang, the deputy researcher, and Qian Liu, the secretary of science and management, and carries out quality control management and progress coordination.
The center will deeply integrate innovative forces such as medical industry, university, research institutes and enterprise, build an efficient full-chain transformation platform for clinical medical experts and academic committee experts, form a new model for the intelligent development of improved new drugs, explore a new path for hospital innovation and transformation, improve the new efficiency of hospital innovation system, and create an innovative intelligent development platform that can truly serve national health and clinical needs.

-END-


转载声明:未经本网或本网权利人授权,不得转载、摘编或利用其他方式使用上述作品。已经本网或本网权利人授权使用作品的,应在授权范围内使用,并注明“来源:新领先医药科技”。

 Hot line:010-61006450
Hot line:010-61006450
 EN
EN

 010-61006450
010-61006450 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450